You are here
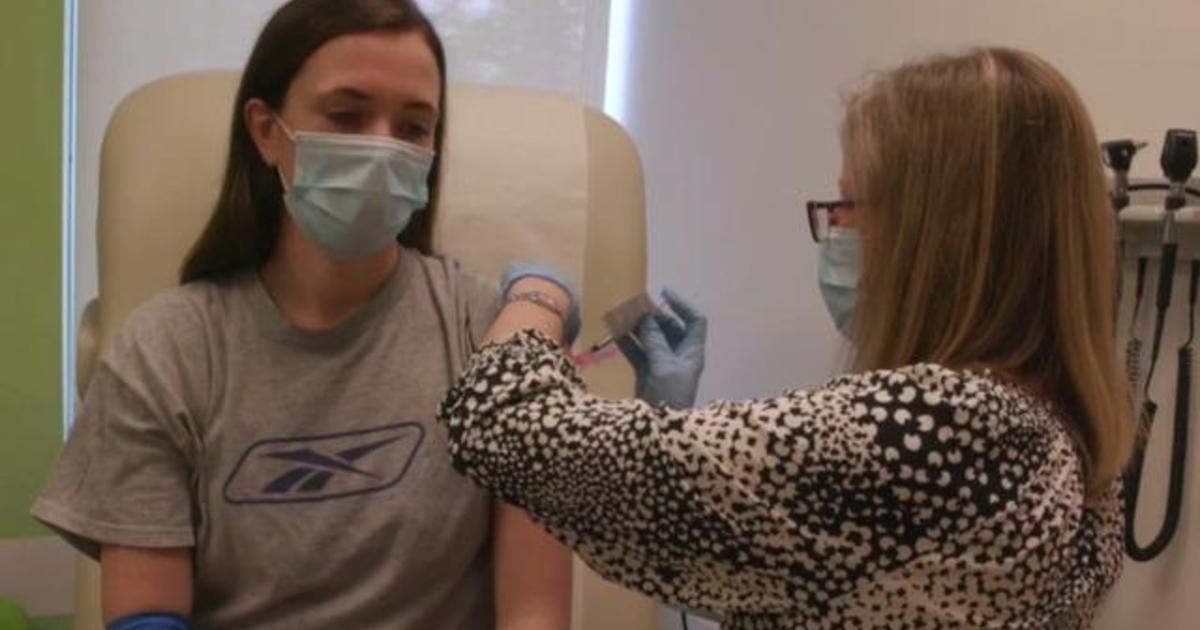 Moderna CEO expects to know in November whether COVID-19 vaccine works More than 25,000 people are enrolled in the drugmaker's late-stage trial of a potential coronavirus vaccine. cbsmoneywatch
Moderna CEO expects to know in November whether COVID-19 vaccine works More than 25,000 people are enrolled in the drugmaker's late-stage trial of a potential coronavirus vaccine. cbsmoneywatch
Moderna, the first pharmaceutical firm to conduct human trials of a coronavirus vaccine in the U.S., said it should know sometime in November whether its vaccine works. Moderna CEO Stephane Bancel told CNBC on Thursday that it could have enough data by October from its late-stage trial to evaluate its efficacy, although he said he viewed that timeline as unlikely, according to the report.
President Donald Trump has asserted that a vaccine could be ready "during the month of October." Experts say that is unlikely given the time required to test and evaluate the vaccines for efficacy and safety. U.S. Centers for Disease Control Director Robert Redfield told Congress Wednesday that a vaccine wouldn't be widely available until the second or third quarter of next year — a projection that Mr. Trump took issue with, saying that Redfield "made a mistake."
So far, there are seven vaccines, including Moderna's product, in final Phase 3 testing, which involves large-scale tests on people.
"If the infection rate in the country were to slow down in the next weeks, it could potentially be pushed out in a worst-case scenario in December," Bancel told CNBC.



Комментариев
Vaccine companies reveal their study designs, even as Trump sows
President Trump stood before a televised audience Wednesday and proclaimed that “results are very good” for vaccines targeting the novel coronavirus. A day later, Moderna and Pfizer, two front-runner drug companies developing a shot, released the full rule books for their studies, revealing that no one yet knows conclusively whether a vaccine is safe and effective — not even company executives....